polarimeter caraway and spearmint|how to calculate polarimetry : maker Caraway seed’s optical rotation is 61o and spearmint’s is -61o. The optical rotation is the ability of a chiral substance to rotate a plane of polarized light. A . Projeto verão Djalma não entende de política, Xandão, Djalma Ferreira, Di Souza. Seleção axé Junior Marques, NEY, Grilo de Roupa, Banda Ojú Obá. Ouça Roubou Meu Coração de Luxúria no Palco MP3 - o melhor lugar para descobrir músicas e artistas novos grátis.
{plog:ftitle_list}
2 de nov. de 2023 · Futebol. Brasileirão Série A, Rodada 31. Cuiabá - Vasco da Gama placar ao vivo, resultados H2H, classificações e previsões. Receber notificações para .

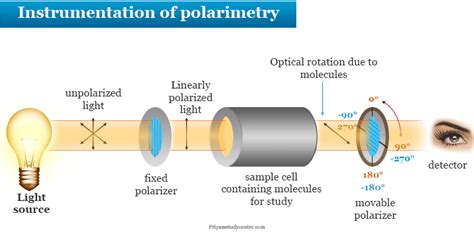
A polarimeter is an instrument used to determine the angle through which plane-polarized light has been rotated by a given sample. You will have the opportunity to use a polarimeter in the .Caraway seed’s optical rotation is 61o and spearmint’s is -61o. The optical rotation is the ability of a chiral substance to rotate a plane of polarized light. A .Overview of the Experiment. You will be given a sample of either caraway oil or spearmint oil. The major component of these oils is carvone. You will separate the carvone from the higher .Specific carvone enantiomers can be isolated in pure form from oil of spearmint or oil of caraway using column chromatography. Limonene, a less polar constituent of these essential oils can .
The plane of polarization can be determined by an instrument called a polarimeter, shown in the diagram below. Monochromatic (single wavelength) light, is polarized by a fixed polarizer next to the light source.Overview of the Experiment. You will be given a sample of either caraway oil or spearmint oil. The major component of these oils is carvone. You will separate the carvone from the higher .2.2.A.a. Analyses to Be Performed on Spearmint and Caraway Oils Odor Carefully smell the containers of spearmint and caraway oil and of the two carvones. Record the impressions. 2.2.A.b. Analyses to Be Performed on the .Ochem help. Polarimetry 1. Which one of the readings for caraway carvone (ave.value from the pure carvone table) was the true value? Explain? 2. Which one of the readings for spearmint carvone (ave. value from the pure carvone table was the true value? Explain? Values for caraway: (positive rotation ) corrected: Clockwise- 54.9 degrees, counter .
In this experiment2 you will be working with oils prepared from caraway seeds and spearmint leaves. Each oil has a distinct and characteristic odor, yet carvone is the major component in both oils! It is amazing that the difference in odor is attributable . and the signs of rotation in a polarimeter. However, some of the physical propertiesReview Topics: Polarimetry (Chapter 17), TLC (Chapter 18), GC (Chapter 20) Terpenes encompass a large family of organic compounds widespread in nature and . caraway, dill, and spearmint in association with other terpenoids such as limonene. Limonene is found in spearmint, caraway, lemon, and orange oils. .
polarimetry and stereochemistry
Right, caraway fruits. Spearmint has an easily identifiable aroma; caraway may not be as familiar, but exhibits a spicier scent and is often added rye breads and sauerkraut. One does not immediately find a similarity between these two plants, but surprisingly, the oils found in spearmint and caraway fruits are both abundant in the chemical carvone.optical rotation of the isolated carvone will be determined by polarimetry. Oil of Spearmint and Oil of Caraway Spearmint Essential oils, derived from natural products, have been used for centuries as flavorings, fragrances and for medicinal purposes. These oils typically contain multiple organic components, generally with one or more(Benches A, B and C will use oil of spearmint and benches D, E and F will use oil of caraway.) Each student will analyze products by TLC, IR spectroscopy, and calculate his or her percent recovery of carvone. Students will then pool their isolated carvone samples for analysis by .essential oil of either spearmint or caraway by column chromatography, a type of adsorption chromatography. The principles of column . Polarimetry Your TA will announce when the polarimeter is equipped with the solution of your oil. Only TAs are to handle polarimeter cells. Measure the optical rotation of your oil and calculate the specific
ORGANIC CHEMISTRY LAB REPORT 3 Polarimetry 02. Professor Singh Chem 76A. PURPOSE. The purpose of this experiment was to get familiar with the polarimeter and optical rotation to identify the specific rotation of spearmint (+) or caraway (-), by determining its specific rotation through optical purity.In this experiment1 you will be working with oils prepared from caraway seeds and spearmint leaves. Each oil has a distinct and characteristic odor, yet carvone is the major component in both oils! It is amazing that the difference in odor is attributable . and the signs of rotation in a polarimeter. However, some of the physical properties
Experiment Report Connor Morris Chem 253 014: Peter Rietgraph Lab: Stereochemistry and Polarimetry Purpose The purpose of this experiment was to extract specific enantiomeric structures that make up caraway seeds and spearmint leaves, and to analyze the similarities of each enantiomers polarimetry and intermolecular forces.A major constituent of oil of spearmint is the R enantiomer of carvone. Minor amounts of limonene, a metabolic precursor of carvone is also present in oil of spearmint. Caraway oil is extracted from caraway seeds (Carum carvi) and contains manily the S enantiomer of carvone along with higher levels of limonene.Experiment 9: Stereochemistry and Polarimetry Purpose The purpose of this lab was to use 4 different tests, TLC, smells, IR, and polarimetry to differentiate the stereoisomers of carvone, spearmint oil (R– (-) carvone) and caraway oil (S- (+) carvone). We also used polarimetry to find the identity of an unknown substance. Theory
In this experiment 2 you will be working with oils prepared from caraway seeds and spearmint leaves. Each oil has a distinct and characteristic odor, yet carvone is the major component in both oils! It is amazing that the difference in odor is attributable solely to a difference in chirality of the carvone in the two oils. . Polarimetry MHS .In both cases the length of the cell is 10 cm = 1 dm. Data collected using the red diode laser (650 nm) and 20 ° C: Material in 100 mm cell p2 (rad) p2 (rad) Air-0.0370-0.0380 Caraway oil 0.651 0.649 Spearmint oil-0.867-0.848 Data collected using the green diode laser (532 nm) ) and 20 ° C: Material in 100 mm cell p2 (rad) p2 (rad) Air-0.0270 .Experiment 14: Spearmint and Caraway oil: (+)- and (-)-Carvones Objective The experiment illustrated the differences between (+)-carvone and (-)-carvone from spearmint oil, using gas chromatography, polarimetry, and infrared .
" OM Volume of caraway carvone plus optically inactive solvent. lO 0 Cell pathlength (in decimeters). / Polarimeter number used in Caraway Carvone Dilution Caraway Carvone Dilution. . Using the true observed rotations for . Experiment 14: Spearmint and Caraway Oil: An Investigation of Enantiomers Experiment # Title Reading in Lab Text Pre-Lab Questions Post-Lab Questions 14 Spearmint and Caraway Oil Essay: Stereochemical Theory of Odor (+) and (-) Carvones Pg. 98-102 Review Technique 25: IR Bruice Text Sections 14.10-14.17 Bruice Text: 22a,b, 23, 30, 31 Technique .
how to calculate polarimetry
Objective: In this experiment, the objective is to obtain samples of (+)-carvone and (-)- carvone, place them through gas chromatography, IR spectroscopy, and polarimetry machines, as well as a odor test. Utilizing the results, we will then compare the (+) and (-)- carvone for odors, retention time, spectra and refractive indices. Introduction: Carvone is terpenoid that is found widely in .
In order to separate the essential oils from the spearmint and the caraway seeds, a steam distillation needs to be performed. The apparatus for a steam distillation (fig. 4) differs from a fractional or simple distillation apparatus due to the addition of a separatory funnel and a claisen head. In this instance, the separatory funnel is used as a dropping funnel to keep the amount .
Carvone is a naturally occurring ketone found in the essential oils of caraway, dill, and spearmint in association with other terpenoids such as limonene. Limonene is found in spearmint, caraway, lemon, and orange oils. Carvone and limonene, both monoterpenes, have only one stereogenic center and can exist in two enantiomeric forms: R and SReview Topics: Polarimetry (Chapter 17), TLC (Chapter 18), GC (Chapter 20) Terpenes encompass a large family of organic compounds widespread in nature and . caraway, dill, and spearmint in association with other terpenoids such as limonene. Limonene is found in spearmint, caraway, lemon, and orange oils. .Next, we did polarimetry. The blank optical rotation was 293.75 o, our unknown was Y, and its optical rotation was 295.50 o, (+) carvone’s optical rotation was 335.75 o, and (-) carvone’s optical rotation was 254.00 o.We found the unknown Y containing 52.1% S and 57.92% R. For the smell test it was not that easy to describe the smells. The S (+) carvone smelt like spearmint leaves . This plant-based chemical is found in a number of growths, especially spearmint and caraway, and it's commonly used to flavor foods. In addition, there's an interesting chemical factoid here: Carvone actually comes in two almost-identical forms, which have different scents and flavors. Welcome to stereo-chemistry.
Lecture reading from TA lab steam distillation of from caraway seeds and carvone from spearmint leaves milan patel february 24, 2016 methods and background the. Skip to document. University; High School. Books; Discovery. . the enantiomers could not be determined with absolute certainty without the use of a polarimetry analysis. However .
Analysis of Spearmint and Caraway Oils 1. Smell the caraway and spearmint tubes, as well as the 2 carvones. . Polarimetry 3. Find the optical rotation alpha of both carvone samples; specific rotation or alpha d can be found from the observed rotation in degrees, the concentration in g per mL of the used solution, the length of the tube that .A polarimeter is an instrument used to determine the angle through which plane-polarized light has been rotated by a given sample. You will have the opportunity to use a polarimeter in the laboratory component of the course. . Carvone from caraway: [α] . Carvone from spearmint: [α] D = –62.5 .
examples of polarimetry
WEBTodas as classificações de Cracovia Krakow em Ekstraklasa, Copa da Polonia para a última temporada
polarimeter caraway and spearmint|how to calculate polarimetry